Affinity Definition - What It Means For You
Have you ever felt a natural pull towards something or someone, a sort of connection that just makes sense? That feeling, that natural connection, is often what we mean when we talk about affinity. It is, in some respects, a very simple idea that shows up in many different areas of life and even in the world around us, from how tiny bits of matter act to how we organize our thoughts. This word, "affinity," describes a kind of attraction or relationship, and its meaning can shift quite a bit depending on where you hear it used, which is quite interesting, you know.
It turns out that this idea of a natural liking or a close connection pops up in some pretty unexpected spots. For instance, it can describe how atoms might behave, or how certain medicines interact with our bodies. It even helps folks in business organize their ideas into sensible groups. So, it's not just a feeling; it's a way to describe how things relate to each other, whether they are big or small, or even just ideas floating around in your head, which is pretty cool, honestly.
This discussion will walk you through what "affinity" truly signifies in several key fields. We will look at its meaning in chemistry, medicine, and even in how people organize information, giving you a clearer picture of this rather important word. You will, like your, get a better grasp of how this concept shapes different parts of our existence, making things clearer for you, so.
Table of Contents
- What's the Big Deal About Affinity?
- Understanding Electron Affinity - A Core Affinity Definition
- Affinity in Medicine - A Different Affinity Definition
- Organizing Thoughts - The Affinity Diagram Explained
- Beyond the Basics - Affinity in Math
What's the Big Deal About Affinity?
When someone mentions "affinity," your mind might first go to a personal liking, like having an affinity for a certain type of music or a specific hobby. That's a good start, but the word actually has a much broader reach, which is quite interesting, you know. It shows up in science, in how we manage projects, and even in the world of numbers. Getting a handle on this word means looking at it from several angles, because it really does mean different things in different places, so.
The core idea, though, remains pretty consistent: it's about a connection or a pull. Whether it's atoms drawing together or ideas grouping themselves, there's always a sense of things belonging together or having a natural tendency to join up. This is what makes the word so useful; it helps us talk about relationships that might not be immediately obvious, but are very real, actually. It's like finding a hidden thread that ties things together, which is pretty neat.
Understanding Electron Affinity - A Core Affinity Definition
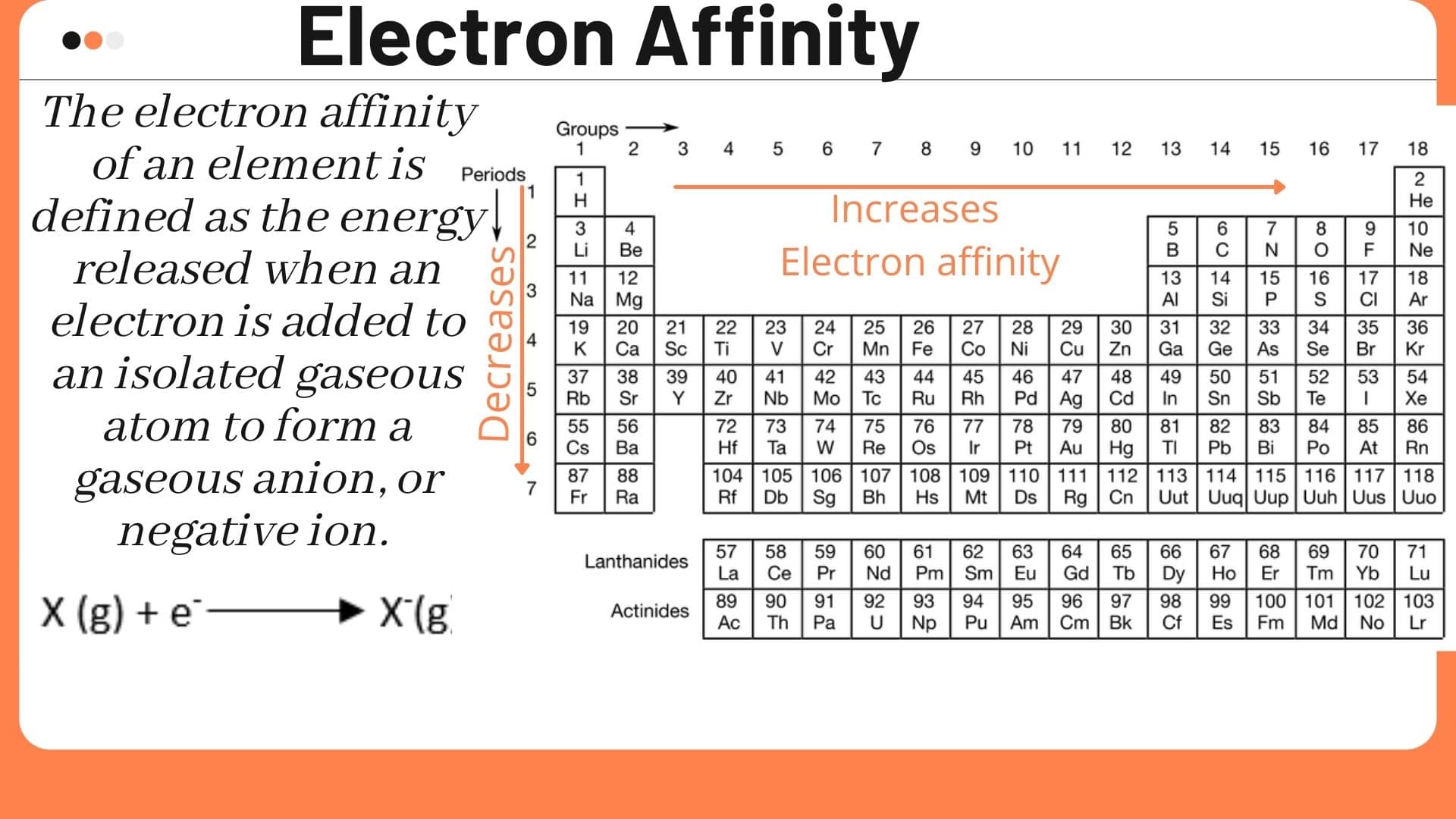
Let's consider the world of tiny particles, where electron affinity comes into play. This idea is about how much energy changes when a neutral atom decides to take on an extra electron, making it a negatively charged particle. It is, in some respects, a measure of how much an atom "wants" an electron, or how happy it is to accept one, which is kind of a funny way to think about atoms, but it works, you know.
When an atom gains an electron, it often lets go of some energy. This letting go of energy is what we call electron affinity. It's a way to measure how much that atom is drawn to an electron. The more energy released, the stronger the pull, or the greater the electron affinity. This is a pretty fundamental idea in chemistry, honestly, helping us figure out how different elements might react with each other, so.
We can also look at how this tendency changes as you move across the periodic table, that big chart of all the elements. Generally, as you go from left to right on the table, elements tend to have a stronger pull for electrons. This is because their atomic structure changes in a way that makes them more eager to fill their outer electron shells. It's a pattern, more or less, that helps scientists predict how elements will behave, which is very useful.
However, like many things in science, there are always a few surprises. Some elements don't quite follow the general pattern. These are known as "exceptions," and they make the study of electron affinity even more interesting. For instance, noble gases, those elements way over on the right side, usually do not want to gain electrons at all, which is pretty much the opposite of what you might expect from the general trend. They are quite content as they are, apparently.
Learning about this particular type of affinity, the electron affinity, can be done rather quickly. You can get a good grasp of the main ideas in just a few minutes. It's all about understanding that energy change and seeing how it fits into the bigger picture of how elements interact. You can even check your grasp of the concepts with a quick practice activity, if you like your, to make sure it all sticks.
How Does Electron Affinity Shape Our World?
This idea of electron affinity, this particular affinity definition, helps us understand a lot about how different materials are put together. It explains why some elements readily form connections with others, creating new substances. For example, it helps us see why salt, which is sodium chloride, forms so easily; the chlorine atom has a strong pull for an electron that the sodium atom is quite willing to give up, so.
It's also pretty important for figuring out how chemical reactions happen. When atoms exchange or share electrons, it's often because of their individual electron affinities. This tells us about the energy involved in making or breaking chemical bonds, which is a big part of how everything around us is built, you know. Without this understanding, our grasp of chemistry would be, well, a little bit fuzzy, to be honest.
Affinity in Medicine - A Different Affinity Definition
Moving from atoms to medicines, the idea of affinity takes on a different meaning, but it still involves a kind of connection. In pharmacology, which is the study of how medicines work, "affinity" describes how strongly a drug binds to a specific target in the body, like a protein or a cell receptor. It's like a key fitting into a lock; the better the fit, the higher the affinity, which is pretty much how it works, apparently.
When a drug has a high affinity for its target, it means it can bind very effectively, even at low amounts. This is a really important quality for medicines because it means you don't need a huge dose to get the desired effect. A medicine with strong affinity is usually more efficient in its work, making it a better choice for helping people feel better, so. It’s all about that snug connection, you know.
We also talk about "potency" in pharmacology. Potency is about how much of a drug is needed to cause a particular effect. A drug that needs only a small amount to produce a big effect is considered very potent. This is where the idea of drug affinity becomes very relevant. A drug with a high affinity often has high potency because it can bind well even when there's not much of it around, which is quite logical, really.
For example, imagine a certain pain reliever. If just a tiny amount of it can stop your aches, it's a potent medicine. This potency is often linked to how well it "sticks" to the pain receptors in your body. That "stickiness" is its affinity. So, the stronger the connection (affinity), the less you need (potency) to get the job done, which is a pretty clear relationship, actually.
You can learn about these medical terms, like potency, efficacy, and affinity, in a fairly quick way, perhaps in just a few minutes. Getting a handle on these ideas is a good step for anyone interested in how medicines do what they do. There are even ways to test your knowledge with a short practice session, if you want to make sure you have a good grip on these important ideas, so.
What's the Link Between Potency and Affinity?
The connection between potency and affinity is pretty direct in many cases. A drug's ability to create a strong effect with only a small amount (its potency) is often a direct result of how well it can attach itself to its intended target in the body (its affinity). If a drug has a very strong affinity, it means it can find and latch onto its target even when there are not many drug molecules present. This makes it very effective, which is quite useful, you know.
So, in essence, a high affinity can lead to high potency. It's like having a key that fits perfectly into a specific lock; you only need one key to open that lock. If the key is a bit wobbly, you might need many keys to finally get the lock to turn. That perfect fit, that strong attachment, is a key part of how medicines work and how we measure their strength, honestly. It's a pretty neat way to think about how our bodies react to what we put into them, so.
Organizing Thoughts - The Affinity Diagram Explained
Now, let's switch gears completely and look at another meaning of "affinity" – one that helps people organize their thoughts and ideas. An affinity diagram, sometimes called an affinity map, is a visual way to sort out a bunch of messy, unorganized ideas. It's used to group these ideas based on how similar they are, or what natural connections they have, which is pretty helpful when you have a lot of scattered thoughts, you know.
Imagine you and your team have just brainstormed a hundred different ideas for a new project. You have sticky notes all over the wall, and it looks like chaos. That's where an affinity diagram comes in. It helps you take all those individual notes and put them into sensible piles. Each pile represents a common theme or category, based on the natural "affinity" between the ideas, which is quite a clever way to bring order to things, honestly.
The process of creating an affinity diagram usually starts right after a big brainstorming session. Once you have a lot of ideas, perhaps written on separate cards or notes, you then start looking for connections. You pick up one idea, then another, and if they seem to belong together, you put them next to each other. This continues until all the ideas are grouped, more or less, into related clusters. It's a hands-on way to make sense of a lot of information, so.
There are typically five steps involved in making an affinity diagram. First, you gather all your ideas, usually from a brainstorming session. Second, you place them randomly, maybe on a large wall or table. Third, without talking, you start moving ideas that seem related next to each other. Fourth, once ideas are grouped, you create a header card for each group that describes the main theme of that group. Finally, you might draw lines or circles around the groups to make them clear. It's a simple, yet very effective, way to get a handle on a lot of information, you know.
For instance, if you're planning a community event, your brainstormed ideas might include "find a park," "book a band," "send out invitations," "get food trucks," "set up chairs," "promote on social media," and "clean up afterwards." Using an affinity diagram, you might group "find a park," "set up chairs," and "clean up afterwards" under a header like "Logistics." "Book a band" and "get food trucks" might go under "Entertainment & Food." "Send out invitations" and "promote on social media" could form a "Promotion" group. This helps you see the bigger picture and plan better, which is pretty useful, honestly.
How Can an Affinity Diagram Help You Grasp the Affinity Definition?
Using an affinity diagram, this practical application of the affinity definition, really helps you see the core meaning of the word in action. It shows that "affinity" isn't just a theoretical concept; it's about things naturally drawing together because they share common traits or purposes. When you group ideas, you are identifying their inherent affinities, their natural connections, so.
It's a very visual way to see how items, even abstract ones like ideas, can have a "liking" for each other, making them belong together. This tool helps you understand that affinity is about finding patterns and relationships that might not be obvious at first glance. It's like sorting a pile of mixed socks into pairs; the socks have an affinity for their matching partner, and you're just revealing that connection, which is pretty much what it's all about, you know.
Beyond the Basics - Affinity in Math
Even in the world of mathematics, the word "affinity" appears, though its meaning here is a bit more abstract. When we talk about "affine functions" in math, we are looking at a type of relationship between numbers that is very similar to, but not quite the same as, a "linear function." It's a subtle but important difference, which is quite interesting, honestly.
A linear function is a straight line that always passes through the origin point (0,0) on a graph. It's like a rule where if you double your input, you double your output. But an affine function, while still making a straight line, does not have to pass through that exact origin point. It can be a straight line that has been shifted up or down, or left or right, from the origin, so.
This means an affine function is basically a linear function that has had a "translation" applied to it. Think of it like taking a perfectly straight line and just sliding it somewhere else on the graph without changing its slope or direction. It keeps its "linearity" in a way, but it's been moved, which is the key difference, you know. This is a bit more complex than the other uses of "affinity," but it still speaks to a kind of structural connection.
For example, if you have a linear function like y = 2x, it always goes through (0,0). If you have an affine function like y = 2x + 3, it's still a straight line with the same steepness, but it crosses the y-axis at 3, not 0. That "+3" is the translation, the shift that makes it affine rather than strictly linear. It's a pretty clear distinction once you see it, apparently.
I was, a bit confused about this difference myself at one point. The main thing to remember is that a linear function is a special type of affine function. All linear functions are affine, but not all affine functions are linear. The added "shift" or "translation" is what gives an affine function its unique quality. Any suggestions on how to explain this even more clearly would be appreciated, you know, as it can be a bit tricky to grasp at first.
Linear Versus Affine - A Look at Another Affinity Definition
The core difference, and thus another affinity definition, between linear and affine functions lies in their relationship to the origin. A linear function is very "tied" to the origin, meaning it always passes through that central point. It has a kind of inherent affinity for the origin. An affine function, on the other hand, does not necessarily share that particular affinity for the origin; it can be anywhere on the graph, as long as it's a straight line, so.
This distinction helps mathematicians describe different kinds of transformations and relationships in geometry and algebra. It shows that even within a seemingly simple concept like a straight line, there are subtle variations that have important implications for how we describe shapes and movements in space. It's all about how closely something is bound to a fixed point, which is a pretty fundamental idea, honestly.

PPT - Serology PowerPoint Presentation, free download - ID:8774
Electron Affinity Definition

Electron Affinity Equation